Experiments on the projection of particles
Prepared Statement of Tesla (For interview with press on 81st birthday observance):
There is one more discovery which I want to announce at this time, consisting of a new method and apparatus for the obtainment of vacua exceeding many times the highest heretofore realized. I think that as much as one-billionth of a micron can be attained. What may be accomplished by means of such vacua is a matter of conjecture, but it is obvious that they will make possible the production of much more intense effects in electron tubes. My ideas regarding the electron are at variance with those generally entertained. I hold that it is a relatively large body carrying a surface charge and not an elementary unit. When such an electron leaves an electrode of extremely high potential and in very high vacuum, it carries an electrostatic charge many times greater than the normal. This may astonish some of those who think that the particle has the same charge in the tube and outside of it in the air. A beautiful and instructive experiment has been contrived by me showing that such is not the case, for as soon as the particle gets out into the atmosphere it becomes a blazing star owing to the escape of the excess charge. The great quantity of electricity stored on the particle is responsible for the difficulties encountered in the operation of certain tubes and the rapid deterioration of the same.
Tesla Cosmic Ray Motor May Transmit Power 'Round Earth -
Brooklyn Eagle - July 10, 1932, John J. A. O'Neill:
"I will tell you in the most general way," he said. "The cosmic ray ionizes the air, setting free many charges—ions and electrons. These charges are captured in a condenser which is made to discharge through the circuit of the motor."

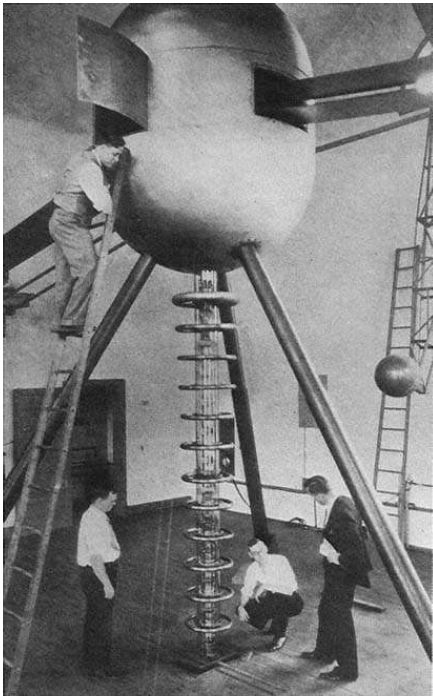
This electrostatic atom-smasher was built at the Carnegie Institution in Washington D.C., and used between 1920 and 1940. The cross-section shows a spherical conductor, its insulating supports, and tube in which particles are accelerated. The charging belt is shown cut-off near the top and bottom. This structure was also the talk of "death-rays."
http://www.photobiology.com/educational/len/
In 1896, one year after Röntgen discovered x-rays, Becquerel observed that the proximity of a photographic emulsion to uranium salts leads to blackening. He classified the emissions according to their penetrating power. Emanations stopped by a sheet of paper were termed alpha-rays (a); those stopped by a millimeter of metal were beta-rays (b); those penetrating a centimeter of metal were gamma-rays (g). In 1909 Rutherford demonstrated that alpha-rays are energetic helium nuclei by trapping uranium emissions in a thin-walled glass vessel and exciting the optical emission spectrum of atomic helium by an electric arc. He showed also that gamma-rays can be diffracted by an ionic crystal and therefore are EMR. The unambiguous identification of beta-rays as fast electrons was finally accomplished in the 1940s by measurements of the charge-to-mass ratio. In 1903 Rutherford and Soddy demonstrated that natural radioactivity is the result of nuclear transformations. An element AXZ is characterized by its atomic number (Z) equal to the number of protons in the nucleus (and the same number of extra-nuclear electrons) and its mass number (A) equal to the total number of neutrons plus protons in the nucleus. The use of Z and the chemical symbol is redundant, although it is often convenient for displaying nuclear reactions. Nuclei having the same Z and different A are isotopes. They belong to the same chemical element and have similar properties, except for the small effects of atomic mass. Nuclei having the same A and different Z are isobars. Isobars usually have different chemical properties, although their nuclear reactions may be similar. A strong attractive force between nucleons is the glue that holds nuclei together. (Nucleons are comprised of quarks which are not relevant to the present subject.) The repulsive force between protons is a destabilizing force. Small nuclei generally have approximately equal numbers of neutrons and proton, e.g.,16O8 has 8 protons and 8 neutrons. Heavy nuclei require more neutrons for stability, e.g., 238U92 with 92 protons and 146 neutrons is radioactive.
Some radioactive isotopes used as sources:
| Isotope | Half life | Principal Radiation Emitted MeV |
| Natural isotopes | ||
| Polonium-210 | 138 days | a, 5.304 |
| Radium-226 | 1620 years |
a 4.777 (94.3%)
a, 4.589 (5.7%) g, 0.188 ( » 4%) |
| Radon-222 | 3.83 days | a, 5.49 |
| Artificial isotopes | ||
| Caesium-137 | 30 years |
b, 1.18 (max)(8%)
b, 0.52 (max)(92%) g, 0.6616 (82%) |
| Cobalt-60 | 5.27 years |
b, 0.315 (max)
g 1.332 g, 1.173 |
| Hydrogen-3 (tritium) | 12.26 years | b, 0.0018 (max) |
| Phosphorous-32 | 14.22 days | b, 1.710 (max) |
| Strontium-90 + Yttrium-90 | 28.0 years (90Y, 64 hours) |
b, 0.544 (max)
b, 2,25 (max) |
| Sulphur-35 | 87.2 days | b, 0.167 (max) |
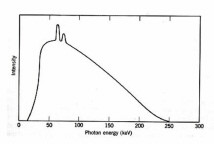
Figure 2. Typical x-ray energy spectrum (Vertical line) for 250 KeV x-rays (horizontal line). The spikes are the "line spectrum"
The x-ray generator invented by Roentgen in 1895 was used almost immediately for medical purposes. X-rays are short wavelength EMR, typically 0.04-0.2 D . [One Dngstrom (D ) equals 0.1 mm.] In an x-ray tube, electrons emitted by a metal cathode are accelerated by a high voltage and strike a metal anode which emits x-rays. An x-ray energy spectrum consists of several sharp spikes superimposed on a continuous background (Fig. 2) The lines are emitted when an accelerated electron ejects an inner-shell electron from a target atom followed by release of an x-ray photon when the "hole" is filled by an outer-shell electron. The continuous x-ray spectrum or bremsstrahlung ("braking radiation") is generated by the rapid deceleration of fast electrons in the vicinity of the target nuclei. The most energetic x-ray photon has an energy equal to eV0 where V0 is the accelerating voltage, e.g., the shortest wavelength x-ray generated by a 100 keV accelerating voltage is 0.12 D for any anode material. The practical energy limit for conventional x-ray tubes is about 300 keV owing to problems of materials and electrostatic breakdown. More efficient devices are available for generation of higher energy photons.
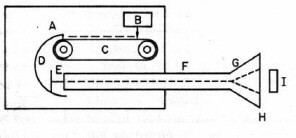
Figure 3. Electron Van de Graaff generator. Electrons from the source (B) are sprayed on the plastic belt (C) and collected by the large metal dome (D). The accumulated negative high voltage on the dome is used to accelerate electrons from the source (E) through an evacuated tube (F). The electron beam is spread by a scanning coil (G), exit through a thin window (H) and irradiate the target (I). Adapted from O’Donnell and Sangster.)
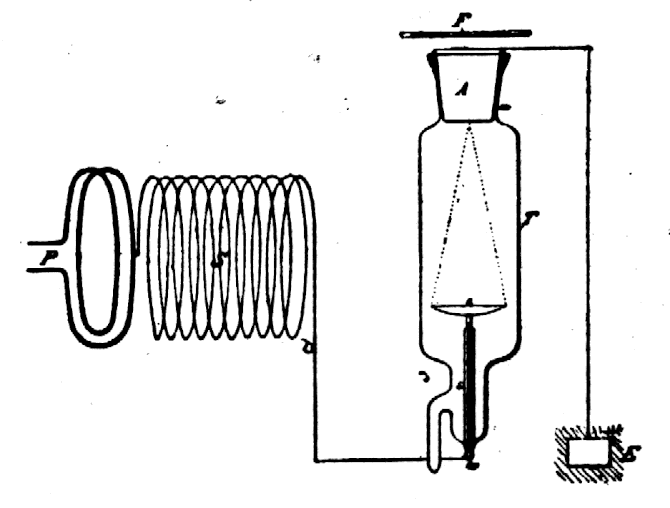
In 1929 Van de Graaff invented an electrostatic machine for acceleration of electrons and heavy charged particles. The Van de Graaff generator is a modern version of Faraday’s famous "ice pail" experiment. A glass rod is charged by friction and touched to the inner surface of a metal pail insulated from the ground. The electric charge localizes on the outer surface of the pail where it does not impede successive transfers of charge from the rod to the inner surface of the pail. Eventually the high voltage on the pail is discharged in the form of a spark. In the Van de Graaff machine the "pail" is a large metal spherical shell charged to high voltage by collecting electrostatic charge from a rotating plastic belt located inside the shell (Fig. 3). The accumulated voltage is used to accelerate electrons or protons generated within a long vacuum chamber located inside the shell. The maximum energy of a conventional proton Van de Graaff generator is limited by atmospheric breakdown to about 20 MeV. A tandem device doubles the useful energy by starting with negatively charged H¯ ions. The ions are first accelerated by the positively-charged shell within an external vacuum chamber, stripped of two electrons by striking a metallic foil, and then further accelerated within the shell as H+ ions.

http://www.nobelprize.org/nobel_prizes/themes/medicine/states/walther-bothe.html
The Van de Graaf band generator, pictured above, was built by Gothe and Wolfgang Gentner in 1936 and was used as a particle accelerator to investigate the nuclear photo-effect in middle weight elements. It was the first accelerator of its kind in Europe.


Other sources to check:
- http://spacewatchtower.blogspot.com.es/2013/01/historic-westinghouse-van-de-graaff.html
- http://www.photobiology.com/educational/len/
- http://neutron.ujf.cas.cz/vdg/graaff-principle.html
- http://www.nobelprize.org/nobel_prizes/themes/medicine/states/walther-bothe.html
- http://oldsite.to.infn.it/activities/schedules/storia/StoriaSezioneTorino_file/wid-e-2002.pdf
- http://publishing.cdlib.org/ucpressebooks/view?docId=ft5s200764;brand=ucpress
 Inventions & Experiments
of Nikola Tesla
Inventions & Experiments
of Nikola Tesla

Write a comment